10 Jun BioMedInnovations LLC Announces U.S. Food & Drug Administration Has Granted Emergency Use Authorization to Manufacture and Distribute SuppleVent™
DENVER, NC – June 10, 2020 – BioMedInnovations LLC (BMI), a medical device company focused on manufacturing precision air and fluid flow devices, with products in development for lung ventilators and ex vivo tissue perfusion, today announced that the U.S. Food and Drug Administration (FDA) has granted its Emergency Use Authorization (EUA) request to manufacture and distribute SuppleVentTM, the Company’s proprietary back-pressure regulator (BPR) based ventilator technology. BMI is working in collaboration with Lawrence Livermore National Laboratory (LLNL) and with partners, Equilibar Precision Pressure Control and Roush Yates Manufacturing Solutions, a division of Roush Yates Engines, to help address the critical and growing need for life-saving healthcare equipment for those experiencing Acute Respiratory Distress Syndrome (ARDS) and other serious breathing difficulties.
“BMI is pleased with the FDA’s decision, which underscores the applicability of the core technology in our organ perfusion system. In partnership with Lawrence Livermore National Laboratory, we were able to quickly design a machine with minimal components that is easy to assemble in an effort to help COVID-19 patients and healthcare providers who are in need of highly efficient, value-added ventilators,” said Sherif Gabriel, chief executive officer of BioMedInnovations LLC.
“This effort has been truly collaborative, and we are honored to have partners such as Roush Yates Manufacturing Solutions ready to quickly manufacture as many as 1,000 of these potentially life-saving machines per week,” said Carrie DiMarzio, chief operations officer of BioMedInnovations LLC.
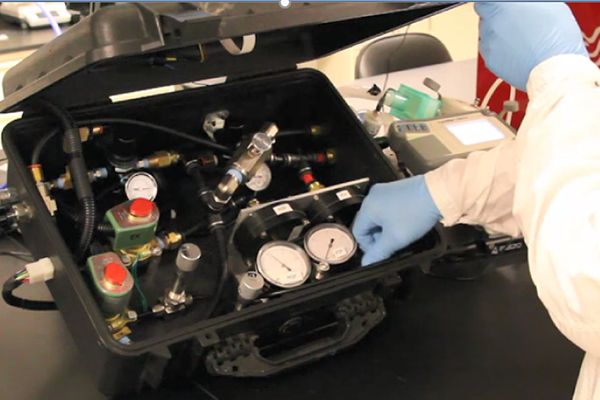
LLNL and BMI pursued a reversed design approach, a methodology guided by anticipated supply chains and ease of assembly. With this mindset, the partnership brought a design forward to full test spanning clinically relevant PIP, PEEP, I:E, RR and FiO2. The machine is a simple assembly of components with an intuitive and clinician-friendly interface that includes full alarms and low power operation from a compressed gas source. Forward flexibility is included in the system architecture to add additional displays, data logging, wireless communication, closed loop control and other features, if desired.
“This is a truly unique system which, importantly, was designed with the provider experience in mind. All of the external components can be quickly sterilized, and internal components have been sourced to ensure biocompatibility with oxygen concentrations,” said Jhamyie Cappiello MS RRT-ACCS RCP, an educator in Respiratory Care Services at Duke University Hospital. “It requires little maintenance, can be rapidly deployed, and is operated following very simple inputs for inhalation and exhalation pressures. The user-interface is a robust touch screen that reports pressure settings and tidal volume, displays waveforms and safety alarms.”
The SuppleVentTM ventilator is designed to accept 50psi compressed air or oxygen mix and is powered by a standard 110V/220V AC outlet or an optional DC battery supply. The device is designed to accommodate a standard 22 mm ventilator circuit outfitted with either a mask or endotracheal tube.
“It is spectacular to have an opportunity to contribute to the collective efforts to address patients in need during this COVID-19 global pandemic,” said Jack Kotovsky, a Lawrence Livermore National Laboratory engineer who spearheaded this ventilator effort. “All of us want to contribute and help individuals in all dimensions of this global pandemic and being able to produce a piece of durable equipment that will directly benefit human life is very exciting. There is still a significant need for ventilators globally. Knowing that we are providing a buildable and scalable design for a high-functioning and value-added ventilator is very gratifying and there is important work to continue to help patients and hospital systems in need.”
When BMI initiated this project, there was significant interest from the world of motorsports. Through BMI’s relationship with Industrial Hard Carbon, the collaborative effort also brought in Roush Yates Manufacturing Solutions, a division of Roush Yates Engines, and NASCAR racing teams, Joe Gibbs Racing, to build components for the ventilator. Indy Car engine designer, Honda Performance Development, also assisted with testing and engineering expertise.
About BioMedInnovations (BMI)
BioMedInnovations LLC is a medical device company focused on manufacturing precision air and fluid flow devices, with products-in-development for lung ventilators and ex vivo tissue perfusion. BMI was founded to challenge the common belief that there are not enough transplantable organs to meet the demand. The Company has redefined the problem as a “shortage of confirmed viable organs available for transplant”—not simply a shortage of organ donors. Understanding this distinction inspired BMI to develop CaVESWave®, which the Company believes is the most significant technological advance in the field of tissue perfusion in the past 40 years.
SuppleVentTM is BMI’s proprietary back-pressure regulator (BPR) based ventilator technology, which was designed to help address the critical and growing need for life-saving healthcare equipment for those experiencing Acute Respiratory Distress Syndrome (ARDS) and other serious breathing difficulties. In June 2020, the U.S. Food and Drug Administration (FDA) granted BMI with Emergency Use Authorization to begin manufacturing and distributing SuppleVentTM to hospitals and clinics, with plans to file for a 510(K) approval.
About Lawrence Livermore National Laboratory (LLNL)
Founded in 1952, Lawrence Livermore National Laboratory provides solutions to our nation’s most important national security challenges through innovative science, engineering and technology. Lawrence Livermore National Laboratory is managed by Lawrence Livermore National Security, LLC for the U.S. Department of Energy’s National Nuclear Security Administration.
About Equilibar Precision Pressure Control
Located near Asheville, NC, Equilibar is an engineering and manufacturing company that specializes in fluid control technology for complex research and industrial applications. Its patented valves and back pressure regulators are uniquely well suited for aerospace, fuel cell testing, pharmaceutical manufacturing, catalysis research and other demanding markets.
About Roush Yates Manufacturing Solutions
Roush Yates Manufacturing Solutions machines high performance engine components to support engine design and development for Roush Yates Engines. Although rooted in motorsports and automotive industries, Roush Yates Manufacturing Solutions has leveraged their passion for CNC manufacturing excellence into the aerospace, defense, medical, and high-tech industries.
We have created a world-class 88,000 sq. ft. facility with over 50 advanced CNC machines located in Mooresville, North Carolina. Roush Yates Manufacturing Solutions is staffed by a diversified professional team from the aerospace, defense, motorsports, power generation, and high-tech sectors from around the United States. Our dedication to quality is exhibited by our commitment to meet AS9100 Rev D certification and ITAR registration standards.
*Courtesy of BioMedInnovations LLC


